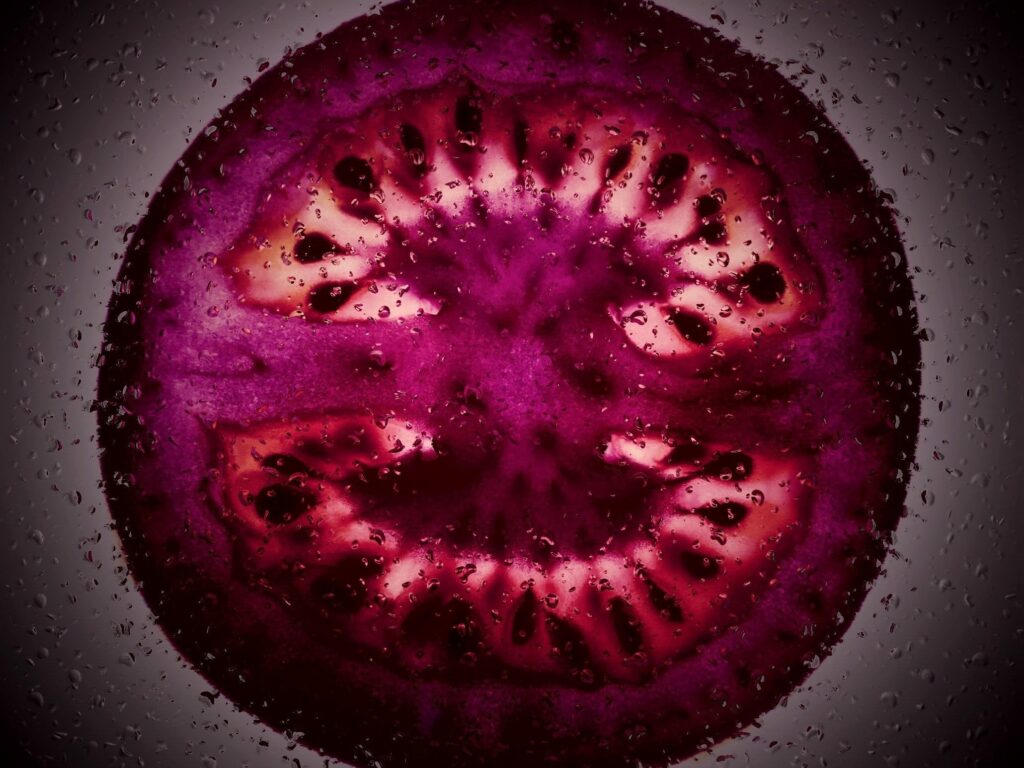
The Edible Object:
On Genetic Craftsmanship with Prof Cathie Martin

The application space for this powerful science is vast, fluid, and still unfolding. In recent decades, it has produced contentious innovations like Golden Rice—developed at the turn of the millennium and later acquired by Syngenta—a genetically modified staple crop biofortified with maize and bacterial genes to address vitamin A deficiency. Touted as a humanitarian intervention, it has also faced criticism for oversimplifying complex nutritional, ecological, and social issues. At the other end of the spectrum is Light Bio’s Firefly Petunia, a bioluminescent flower engineered with genes from mushrooms. Marketed as a ‘designer plant’, it signals a shift from GMOs as necessity to GMOs as lifestyle—where genetic modification becomes an expression of taste as much as science.
As lab-grown biotechnologies take root, they challenge us to reconsider how we engage with the living world—where the boundaries between human and more-than-human are not fixed, but permeable, and often illusory. How might we design with this entanglement in mind?
Natsai Chieza meets with geneticist, plant biologist and innovator Professor Cathie Martin, co-founder of Norfolk Plant Sciences—the Norwich-based team behind the Purple Tomato, a gene-edited food crop infused with snapdragon DNA, now available to North American home growers. Together, they shed light on the expanding space of food design—the scientific, cultural, regulatory, and neuroaesthetic conditionality surrounding genetically engineered plants—and their place on our allotments, our plates and our palettes.
Cathie began her career as an organismal biologist, fascinated by plants and nature with a researcher’s deep curiosity, seeking to uncover truths yet to be known. ‘I spent a lot of time doing field botany, looking at different species, trying to understand why they grew in certain soils. It enriches your understanding of the world; it’s like walking through a beautiful national park and knowing why pine trees grow in one area but not another, or why certain flowers bloom in the desert at specific times. It adds an extra level of insight, and you can truly appreciate nature in a deeper way.’
With a Phd in plant biochemistry, Cathie arrived at the John Innes Centre—one of the UK’s leading research institutions responding to fundamental questions in plant sciences, genetics and microbiology—to study genes that encoded enzymes responsible for pigment production. But it was Cathie’s own gene of ‘surgical’ curiosity that led her deeper into the metabolic lives of plants. Seeing them as extraordinary chemists—producing pigments not just for appearance but with purpose—she began exploring the functions and interconnections of these compounds within the plant, and their potential benefits beyond it. This fascination ultimately led her to not only study but also clone plant genes: work that laid the foundations for the purple tomato.
For NPOL, Cathie tells the behind-the-scenes story of this gene-edited fruit designed to appeal to both eye and body. From exploratory laboratory research to home-grown edible crop, the path has been far from linear—revealing along the way how it quietly mirrors our complex relationship with nature and the systems we’ve created around it.

NOT YOUR USUAL RECIPE
Natsai Chieza: It’s a real privilege to be able to discuss the purple tomato with you given your proximity to the project. Can you tell us the story of how it came to be?
Cathie Martin: Before modern gene editing [t. - 1] tools and biotechnology, we used transposable elements to mutate and clone genes, helping us understand gene function in the species we studied. Think of them as mobile bits of DNA that can jump around the genome. They’re able to cut themselves out of one part of the DNA and insert themselves somewhere else—sometimes causing certain genes to switch on or off, or changing how those genes are regulated. When I started at the John Innes Centre, I worked on mutagenesis [t. - 2] studying genes involved in pigment production—specifically, the enzymes responsible for making anthocyanins, which are the compounds that give many plants their red, purple, or blue colour.
I got involved in an EU-funded project on tomatoes, where the goal was to increase the antioxidant capacity of tomatoes to make them healthier. While there wasn’t hard evidence at the time, there were strong suggestions that fruits rich in anthocyanins—natural pigments found in cranberries, blueberries, blackberries—had health-promoting properties. That benefit might have come from their antioxidant capacity, or from some other mechanism, but either way, anthocyanins were the common factor—and that was the pathway I knew how to manipulate.
Domesticated tomatoes don’t usually produce anthocyanins in the fruit, though some wild species do in the skin. We engineered tomatoes to produce these pigments in the fruit itself—and it worked. The purple phenotype [t. - 3] was incredibly dramatic.
A key technical breakthrough was using a natural fruit-specific promoter [t. - 4] already present in tomatoes—a kind of genetic switch that only activates in the fruit. That allowed us to avoid the typical pitfalls of synthetic biology approaches; we didn’t need artificial or inducible promoters. Tomatoes naturally produce these pigments in their leaves under stress, like drought, but making the pigment throughout the plant can harm growth. In fact, we’d seen this in tobacco—plants turned so purple they couldn’t photosynthesise well, looked etiolated, and were basically sick. By timing anthocyanin production to occur only during fruit ripening, we avoided negative effects on the plant’s growth and yield.
Even though the results were striking, we couldn’t publish in scientific journals at first—it wasn’t of interest without evidence of health benefits. So we teamed up with colleagues at the European Institute of Oncology in Milan where they did animal experiments using p53-knockout mice [t. - 5] , which are cancer-prone and commonly used in nutrition research. We supplemented their diet with pellets containing 10% of our purple tomatoes—and the results were significant. The mice lived 30% longer than those given red tomato supplements. This was the first experiment using isogenic foods [t. - 6] to directly attribute a health benefit to anthocyanins, rather than a vague extract like cocoa powder or chocolate. That paper finally got accepted in 2008, and the story took off globally. I was contacted by people from all over the world asking, ‘Can I get some of those tomatoes?’ And at the time, all I could say was: eat lots of berries!
My experience with getting the purple tomatoes commercialised, and now thinking about new products that we're pushing through, has made me realise that the biotechnology behind it— the trait that you engineer—is only a tiny proportion of what you need to do to make a product successful.
Natsai Chieza: How did your experience navigating the commercialisation of genetically modified crops reshape your understanding of what it actually takes to bring biotech innovation into the world—and what does that say about the gap between scientific ideals and real-world impact?
Cathie Martin: I think the most important moment was when people started asking: ‘Where can I buy it?’. That made me feel strongly that we need to take it a step further. So we set up the next stage. Jonathan Jones [t. - 7] and I began working together—he wanted to get a blight-resistant [t. - 8] potato to market, but was told it would have to be a genetically-modified strain with a ‘consumer trait’ associated with it to gain acceptance in Europe. So he asked me if I could add one. We chose to make high-flavanol potatoes rich in antioxidants over ones with red or purple flesh since those colour varieties already exist in nature. But after a year, it became clear that neither blight resistance nor the consumer trait we had chosen was compelling enough—at least not in potatoes. The public just didn’t want GMO [t. - 9] potatoes regardless. Now I’m actually working on a project promoting health benefits of red flesh potatoes, which are rich in anthocyanins, but these are non-GMOs, so I’ve come full circle.
My experience with getting the purple tomatoes commercialised, and now thinking about new products that we’re pushing through, has made me realise that the biotechnology behind it—the trait that you engineer—is only a tiny proportion of what you need to do to make a product successful. Take a tomato, for example: it has to look great, it has to taste great, and it can’t cost much more money than a regular tomato would. For all of the nutritional traits, there’s an awful lot of work to go from what you produce in the lab, which is not edible or attractive in any way at all, to producing something people will grow or buy. It’s important to recognise that there had to be a lot of continuous design improvement that went into that evolution.
Still, that experience really shaped my perspective on how as academics, we often hold ourselves to ideals—we say we don’t want to make money, we just want to do pure science. But I realised that if you develop something that genuinely has a potential to help people, then you have to try and commercialise it. There is no other way to get into the world. It took us a long time to reach the point where people could actually buy it—and that process was a huge learning curve in itself.


Then we hit a major hurdle: in order to bring a genetically modified crop to market, you need to go through a full regulatory approval process. In the US, that can cost around $100 million and take many years.
FROM LAB TO ALLOTMENT: What It Really Takes to Bring Biotech Pants to Market
Natsai Chieza: Can you elaborate on the other factors beyond lab research that need to align for a biotech innovation to move toward real-world impact? How did your team navigate these challenges with the purple tomato project, especially in light of what will have been extremely challenging regulatory and commercial hurdles?
Cathie Martin: We continued working on the potato project for about a year, but once it became clear that potatoes weren’t the way forward, we pivoted back to purple tomatoes. Around that time, we received some excellent advice—particularly from Eric Ward [t. - 10] , who had been visiting the Sainsbury Lab [t. - 11] , and had deep biotech experience. He encouraged us to treat the tomatoes not as a new idea, but as a product with real potential. We already had the animal study that supported their relevance to human health, so we weren’t starting from scratch. From there, we deepened our research in collaboration with others. We were able to show that anthocyanins in the tomatoes could alter the gut microbiota in animals, and might even mitigate inflammatory diseases in the gut. So we started building a portfolio of evidence around these isogenic foods, demonstrating their clear links to health benefits.
Then we hit a major hurdle: in order to bring a genetically modified crop to market, you need to go through a full regulatory approval process. In the US, that can cost around $100 million and take many years. That was obviously daunting. At the time, one of our advisors suggested a different path. Instead of launching with seeds or growing plants commercially straight away, we could start by producing a seedless juice, which would minimise environmental concerns and simplify the regulatory pathway. And in the US, we could still grow tomatoes under field trial conditions. That became our working model for quite some time.
Natsai Chieza: So when they said, ‘make a product’, what they meant wasn’t the raw material—the raw tomato—but the value-added product, such as tomato juice?
Cathie Martin: You grow it as a ‘field trial’ which doesn’t cost $100 million dollars. That was the model we followed, and at the time, it was also being used by a few other smaller biotech companies, like Arcadia [t. - 12] . They were developing oilseed crops with improved lipid profiles and selling the derived products while growing the plants under field trial conditions. I’m not sure if they’re still using that approach, especially given all the regulatory changes since then, but at the time, it was a workable path forward. So we adopted a similar strategy.
Eventually, we decided to pursue notification with the US Food and Drug Administration (FDA) [t. - 13] . It’s a voluntary process but it carries weight, because the FDA can evaluate whether a product is safe to consume. When I say “we went for it,” what I mean is—I had to write this enormous, complex document and as an academic, I had no clue how to approach the process. The FDA is much more accustomed to working with big companies that know the ins and outs of regulation—there’s not even an actual application form. They refer to it as the Biotech Notification File, but it’s not a form in any real sense. It’s abstract, not even virtual—you can’t just go online and fill it out. It ended up taking me four years to gather all the required data, addressing things like nutritional composition, whether the tomatoes contained any novel proteins, and if so, are they digested quickly or could they be potential allergens?
The FDA were actually very supportive; I think they genuinely wanted to see the product approved, but the process was slow. One of the most frustrating moments came after we had completed a full nutritional analysis of the tomato juice. They came back and said, ‘No, it has to be the whole fruit. For safety reasons, we need to assess the entire tomato, not just the juice.’ So we had to do the whole nutritional analysis all over again—this time using the full fruit!
Natsai Chieza: That thesis, that it didn’t have to be about the tomato itself, but rather the value-added product, raises the question: how much power does regulation have over what we can design—or even imagine—with biotechnology?
What I really like about our approach with the purple tomatoes is that we’ve made them available to home growers in the US. We've really tried to play into the consumer angle—saying, ‘Grow these at home, and if you don’t like them, that’s okay.’ We have so much confidence in them that we’ve not protected them with patents; they’re available as seeds or as plants in local garden centres or farmers’ markets.
Cathie Martin: In hindsight, it was actually quite useful that we had to go back and redo the analysis on whole fruit. Around the same time, US regulations for genetically modified crops were updated to make the process faster and more accessible, especially for small companies like ours. The US Department of Agriculture (USDA) launched a new system called the Regulatory Status Review [t. - 14] , aiming to simplify things.
We acted quickly and submitted our data through this new pathway. Not long after, our purple tomato became the first product approved under the updated rules. Even better, the approval covered not just that tomato, but the method we used to make it—meaning we could use the same technique again without starting over.
But in late 2024, a court case challenged the USDA’s process. Some farmers’ unions and environmental groups said the agency had relaxed its standards too much, specifically around pesticide and chemical application criteria. The court agreed, and the new approval system was paused. Now, things are in limbo again. I hope the decision will be appealed, but with the current political climate, it’s hard to know what comes next.
Natsai Chieza: This is really fascinating and underlines how regulation fundamentally drives the adoption of these technologies and shapes the frameworks around them. I’m trying to avoid getting too technical for our creative audience, who might not usually think about this, but it’s crucial to surface: what needs to be true at the molecular level for great design, how that translates into real consumer value, and how it’s ultimately packaged. Regulation is key—not only for building confidence to invest and scale but also for enabling research institutions and other stakeholders to commercialise innovations and deliver meaningful impact.
There’s a clear tension here—something we’ve seen with AI and consumer biotech alike—where the UK excels at research but struggles to scale technologies. Regulation plays a large part in that, among other factors. So I’d love to hear your perspective on why a technology designed and stress-tested in the UK often can only scale effectively in the US.
Cathie Martin: In the UK, attitudes towards GMO foods can be quite different, and the regulatory environment does not allow us yet to sell either the tomato plant, nor the seeds. However, in the future, we might focus solely on small-scale growing and see what people think.
What I really like about our approach with the purple tomatoes is that we’ve made them available to home growers in the US. We’ve really tried to play into the consumer angle—saying, ‘Grow these at home, and if you don’t like them, that’s okay.’ We have so much confidence in them that we’ve not protected them with patents; they’re available as seeds or as plants in local garden centres or farmers’ markets.
It’s working well so far, especially in the US, where we’re exploring purple tomatoes as ingredients in salsa production. To grow them at a large scale, there is still breeding work to do, like adding virus resistance, so that farmers can grow them more reliably.


SCALE AT THE SPEED OF TRUST
Natsai Chieza: That’s the thing, food cultures vary so much by place, and provenance plays a huge role. While it’s not always the dominant mindset, the idea of locally sourced, field-to-fork food is definitely thriving, with consumers craving transparency about where their food comes from. So when you talk about ‘home growers’, who exactly do you mean? Are you referring to everyone—from small-scale farmers to people growing a few plants on their windowsills?
Cathie Martin: In the first instance, I wanted the seeds to be available for people to grow at home. When we sold them to home gardeners, the response was really positive. You can save your own seeds and replant them—no one is going to charge you extra down the line. The seeds are inbred, so they won’t lose their purple trait when you grow them again. I think that helps reassure people that even though it’s GMO, there are real benefits. Maybe it’s because I’m an academic, but I’ve always believed in giving people a chance to grow things themselves. Some people write to us asking for seeds—not always with a reason, just because they want to try—and I think that’s great. If these tomatoes can help even one person eat more fruit and veg, then it’s worth it.
Natsai Chieza: It’s such a fascinating, brave thing to say, ‘We want to start with the consumer.’ Take for instance the legacy of Monsanto: Monsanto assumed it was business-to-business. And so it was only ever a conversation between the company and the farmer, and that’s where trust is broken, where the consumer isn’t part of that journey. I wonder what that dialogue looks like in your mind?
Cathie Martin: When I go to a supermarket or a market stall, I’m always drawn to the fresh fruit and vegetables. If something looks novel and appealing, that’s what draws me in. I want my products to look good. Home growers are fantastic because they, too, want novelty. They take pride in growing something a bit different, whether it’s a bigger bean or a tastier tomato. So, I’d say that drive towards a small-scale, consumer-focused orientation probably comes from me. I’m also the only woman on the team, and my husband jokes that I’ll buy anything if it looks good!
Natsai Chieza: You used the term ‘consumer trait’ earlier, and talked about going direct to consumer, but what you’re really doing is connecting with a culture that growers share—one that is about the novelty, the benefits, and, at that scale, the pure joy of gardening.
People often ask, ‘Would you actually eat one of these tomatoes?’ And the answer is absolutely yes. We made sure they taste really good.

Cathie Martin: I get that from my father. I remember him—he has long since passed—looking through a magazine or a seed catalogue and seeing these giant potatoes you could buy and grow. He ordered them because they seemed interesting. But it wasn’t that the potatoes were bigger—you just had to grow them in an upright mound, and he was so disappointed. I’m very much like him in that way—I love trying new things too.
For me, the real way forward is to truly believe in what you’re doing. People often ask, ‘Would you actually eat one of these tomatoes?’ And the answer is absolutely yes. We made sure they taste really good.
Natsai Chieza: What do you say to those who are sceptical about GMOs, especially given the legacy of industries today in 2025 and with the tech stack that exists now, we’re just not in the same place we were before. What’s the message to people for whom the red line around any kind of genetic modification isn’t so much about materials, or divesting from fossil fuels, but actually about the food they eat?
Cathie Martin: I think it’s important that people have the freedom to choose whether or not to try GMOs. If someone doesn’t want to, that’s absolutely fine—but a lot of people don’t have an issue with them. I was on the Royal Society [t. - 15] panel around 2016 for a survey that explored public attitudes towards GMOs. Most hadn’t really thought about GMOs before. Some looked them up online minutes before the interviews, some expressed outdated concerns. But once the technology was explained clearly, most people were open to it. One lady in the over-65 group said, ‘Well, if it could stop caterpillars growing on my cabbages, that would be wonderful!’
That stuck with me. People weren’t ideologically opposed—they just wanted solutions to practical problems. I think a lot of resistance to GMOs in the UK is left over from the early 2000s debates. Today, I’m not sure it’s a contentious issue anymore.
As the field expands, more questions reveal themselves about the values embedded at the core of these technologies and the potential for citizens to guide their future embodiment. While the dominating concerns about GMOs from the previous millennium may feel outdated, all sorts of downstream consequences continue to ripple through the present—many of which are yet to be defined as we move along the continuous thread of technological novelty.
From food to flora, from health to aesthetics, we are now witnessing the emergence of a new design space, where genetic modification becomes not only a response to necessity but an articulation of desire, identity, and imagination. In such a cultural container, as Cathie highlights, the imperative doesn’t live solely in monetary value, nor in product affordances such as nutritional benefits, as in the case of the tomato. The shift in how we relate to life as a medium, asks us to look beyond the economic and scientific vision, to how we generatively integrate innovation: What does it take to embed these products in existing systems in ways that amplify ecological balance and long-term resilience? How do we spread benefits in a way that’s ethical? And how do we take communities along on that creative journey?
The story of the Purple Tomato, as well as its predecessors, reveals that ultimately, good stewardship of these transgenic creations requires not only humility, but the quiet, attuned curiosity of a true field botanist—one who tends to the spirit of a place, from the hidden life of the rhizosphere to the shifting canopy above, seeking to fathom the entanglement of time, life, and matter.
Cover image by jj01.
